Disclaimer
The alfapump® system is currently not approved in Canada for commercial use. DSR® therapy is still in development and is currently not approved in the United States or Canada. Any statements regarding safety and efficacy arise from ongoing pre-clinical and clinical investigations which have yet to be completed. Sequana Medical makes no claims of safety or effectiveness of the DSR® therapy in the U.S. or Canada. There is no link between the DSR® therapy and ongoing investigations with the alfapump® system in Europe, the United States or Canada.
Our alfapump® is one of the first medical devices designed to treat the build-up of fluid in the abdomen. It is a battery-powered pump that is implanted under the skin for the controlled and continuous removal of fluid from the peritoneal cavity into the bladder where it is simply urinated away. The alfapump® system provides an automated system for the removal of fluid without the need for repeated needle punctures, needles or external tubes.
The alfapump® has received CE Mark approval for refractory liver ascites. In January 2019, the US FDA granted the alfapump® Breakthrough Device designation for the treatment of recurrent or refractory liver ascites.
We submitted a Premarket Approval (PMA) application to the US FDA in December 2023 having reported positive primary and secondary endpoint data from the North American pivotal POSEIDON study of the alfapump® in recurrent or refractory ascites due to liver cirrhosis.
In December 2024 alfapump® received U.S. FDA Approval and is the first U.S. approved active implantable medical device for the treatment of recurrent or refractory ascites due to liver cirrhosis.
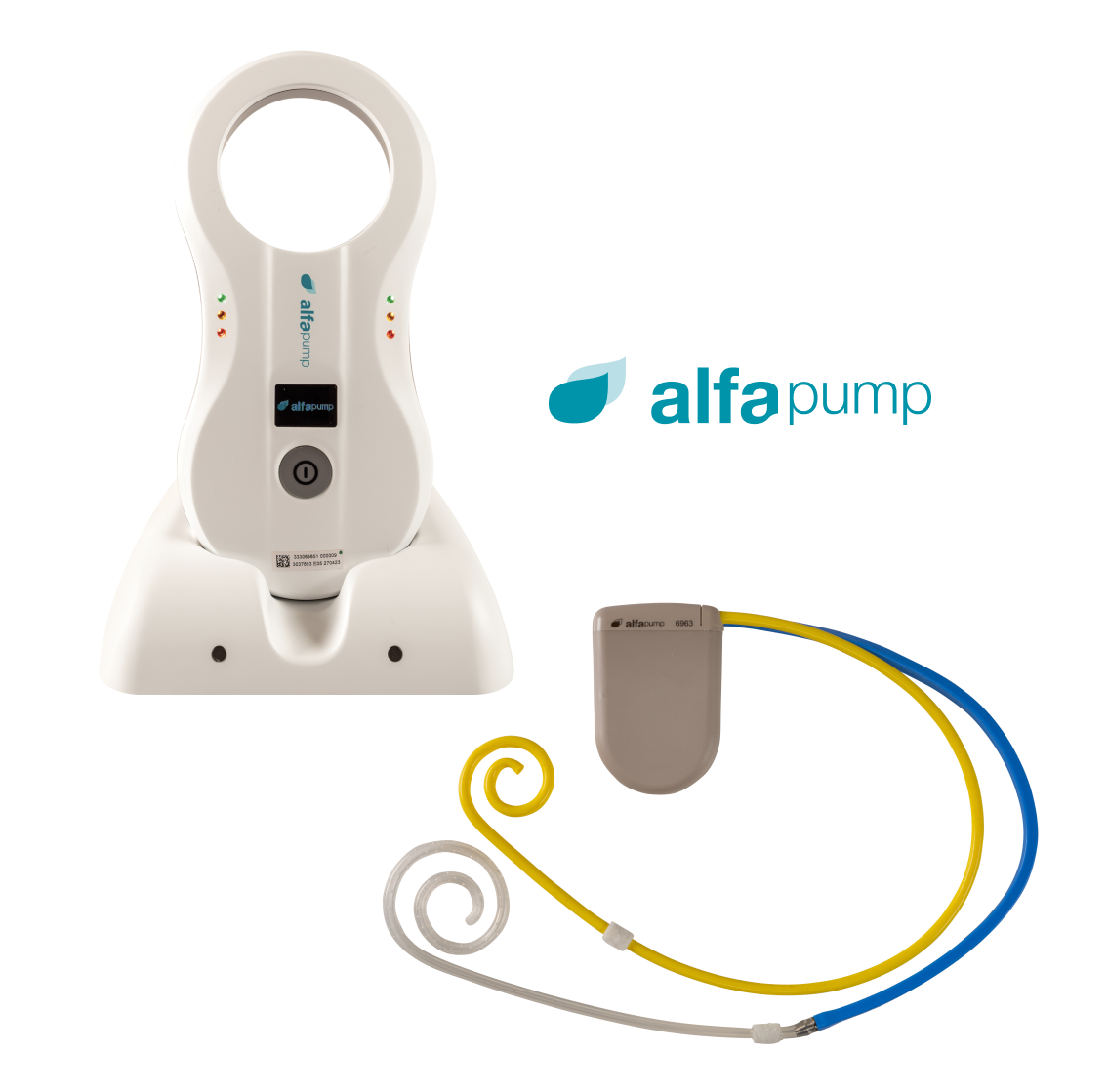
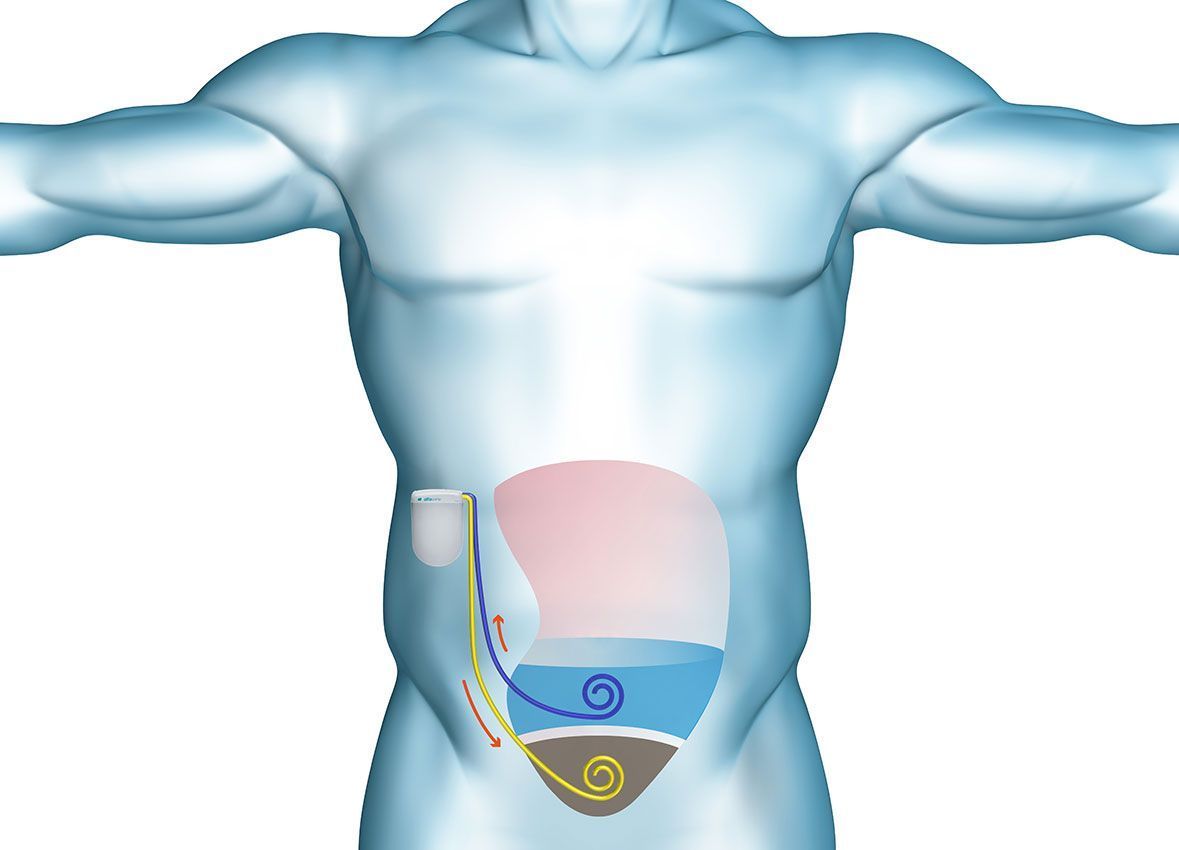
The alfapump® is implanted under the patient’s skin in a minimally invasive operation. It is a simple procedure taking approximately 80 minutes that can be performed under local anaesthesia with sedation, by interventional radiologists. Because the alfapump® is fully implanted, patients are able to retain normal mobility and activity. Once the alfapump® has been implanted, it is programmed wirelessly by the physician to allow the defined amount of fluid to be removed each day. The schedule can be designed to suit patients’ individual daily routine.
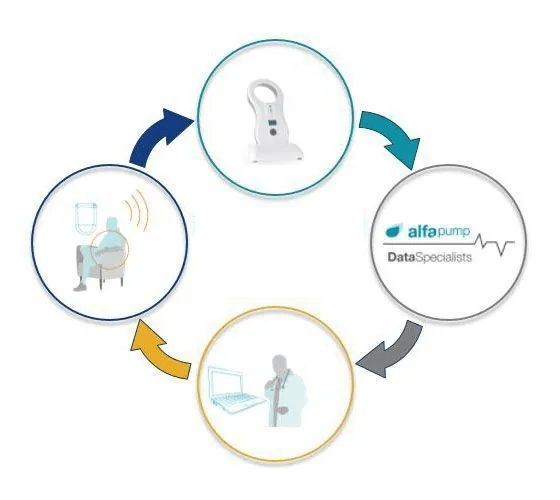
The only patient interaction is the need to recharge the battery each day with a wireless charger (the Smart Charger) through the skin for approximately 30 minutes (depending on the amount of fluid extracted each day). While charging, pump performance data from the alfapump® is transferred to the Smart Charger.
alfapump® performance data is collected and transmitted to secure servers for analysis. Our data specialists receive pump performance information (e.g. volume pumped and pump charging) and report this information to clinicians enabling them to manage patients more effectively through closer monitoring and notification of changes in pump performance data.
We have invested significant resources in clinical studies in Europe and the US to demonstrate the safety and efficacy of the alfapump® in patients with recurrent or refractory liver ascites.
The PMA application was submitted to the US FDA in December 2023 and was based on the successful execution of our pivotal POSEIDON study, a landmark study across 18 centers in the US and Canada with a total of 69 patients implanted with the alfapump®. The primary effectiveness endpoints at six months post-implantation in the Pivotal Cohort exceeded the predefined thresholds with statistical significance, and primary safety endpoint data was in line with expectations [1], [2]. Data at 12 months post-implantation continued to show a strong and durable clinical profile, virtually eliminating [3] the need for therapeutic paracentesis and delivering a clinically meaningful improvement in patients’ quality of life. Sequana Medical reported promising 24-month results from the POSEIDON study presented at the AASLD Liver Meeting in November 2024, showing that the alfapump® system effectively controls ascites and significantly reduces the need for large volume paracentesis (LVP) in patients with refractory ascites [4].
[1] https://journals.lww.com/ajg/abstract/9900/the_effects_of_alfapump_on_ascites_control_and.1522.aspx
[2] https://www.sequanamedical.com/press-releases/sequana-medical-announces-data-on-alfapump-safety-and-quality-of-life-to-be-presented-at-easl-congress-2024/
[3] Based upon 100% median reduction in therapeutic paracentesis in the POSEIDON pivotal cohort, data reported in press release of 19 October 2023.
[4] Based upon the pivotal cohort of the POSEIDON study, data reported in press release of 18 November 2024
Positive top-line data from POSEIDON were reported in the press releases on 25 October 2022, 19 October 2023 and, 18 Novemeber 2024.