Disclaimer
The alfapump® system is currently not approved in Canada for commercial use. DSR® therapy is still in development and is currently not approved in the United States or Canada. Any statements regarding safety and efficacy arise from ongoing pre-clinical and clinical investigations which have yet to be completed. Sequana Medical makes no claims of safety or effectiveness of the DSR® therapy in the U.S. or Canada. There is no link between the DSR® therapy and ongoing investigations with the alfapump® system in Europe, the United States or Canada.
Direct Sodium Removal or DSR® has the potential to break the negative feedback cycle of cardiorenal syndrome, through effective control of the volume status for an extended period of time and thereby avoiding the negative consequences of loop diuretics.
Direct Sodium Removal (DSR®) therapy is our novel and proprietary approach under development for the management of fluid overload due to heart failure.
The sodium concentration in patients with fluid overload is in balance but there is too much sodium and too much fluid in the body.
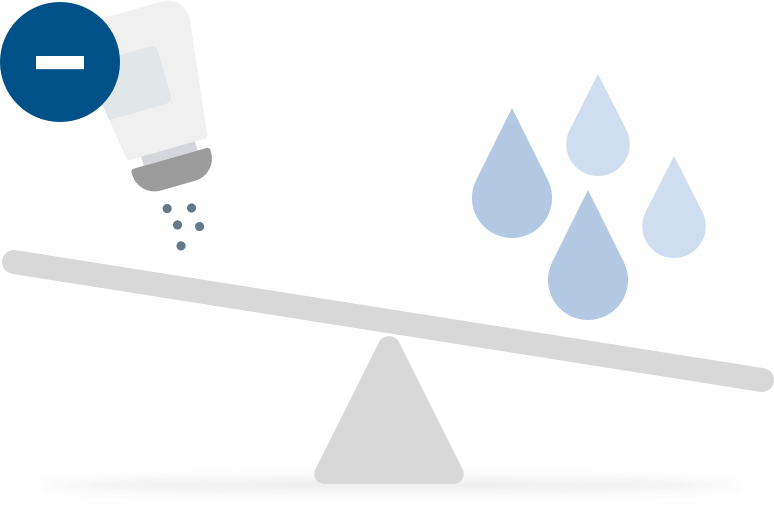
Our approach is to remove excess sodium in patients with residual renal function.
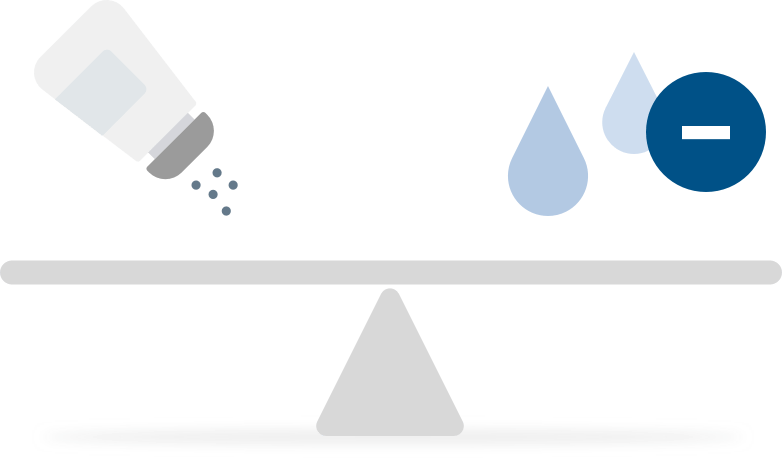
As a result, the body acts to restore the sodium concentration in the body by eliminating fluid through urination and osmotic ultrafiltration, resulting in a sustained level of fluid loss.
DSR® therapy involves the use of the peritoneal cavity for the removal of sodium via diffusion. To do this, a “sodium-free” solution known as “DSR® product” is administered into the abdomen and allowed to dwell for a pre-defined period.
During this time, sodium diffuses from the body down a steep diffusion gradient into the DSR® product. Circulation keeps the effective blood sodium concentration high. The DSR® product and the extracted sodium are then removed, resulting in a removal of sodium from the body. The body responds by eliminating the associated fluid via osmotic ultrafiltration (the movement of water, together with sodium, from the bloodstream to the peritoneal cavity) and/or urination.
The impact of administering a sodium-free DSR® product into the peritoneal cavity, and the resulting sodium and fluid removal, was evaluated in pre-clinical and clinical proof-of-concept studies using single and repeated dose DSR® therapy.
Proof-of-concept data from a pre-clinical and first-in-human single dose DSR® study have been published in the high impact clinical journal Circulation.
Positive top-line data from RED DESERT, a repeated dose DSR® study in 8 euvolemic heart failure patients on high dose diuretics, were reported in the press release and webcast on 11 May 2021. Results were selected for the Late Breaking Science Results at the Heart Failure 2021 Online Congress.
Positive top-line data from SAHARA, a repeated dose DSR® study in decompensated diuretic-resistant heart failure patients, were reported in the press release on 15 November 2022.
Data from the RED DESERT and SAHARA studies have been published in the prestigious peer-reviewed journal European Journal of Heart Failure.